“We’ve received our no objection certificate, and we’ll be initiating our clinical study in India soon. We’ll try to move through the regulatory process as soon as possible,” Dion Warren, head of India and Southeast Asia Multi-country Organisation, Takeda Pharmaceuticals, said in an interview to ET on the sidelines of BioAsia2024 in Hyderabad.
India’s drug regulator has laid a precondition of conducting local bridging study for approval of the jab.
Takeda Pharmaceuticals’ dengue vaccine assumes significance since the first vaccine ever licensed for dengue, developed by French drugmaker Sanofi Pasteur, met with failure as it only helped people who had been previously infected with the virus.
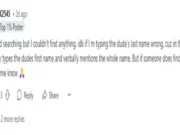
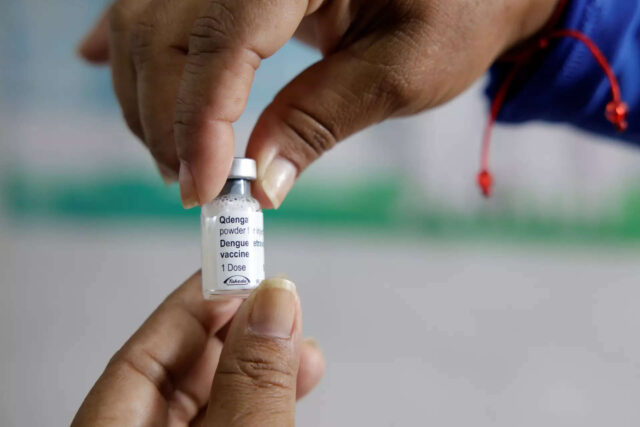
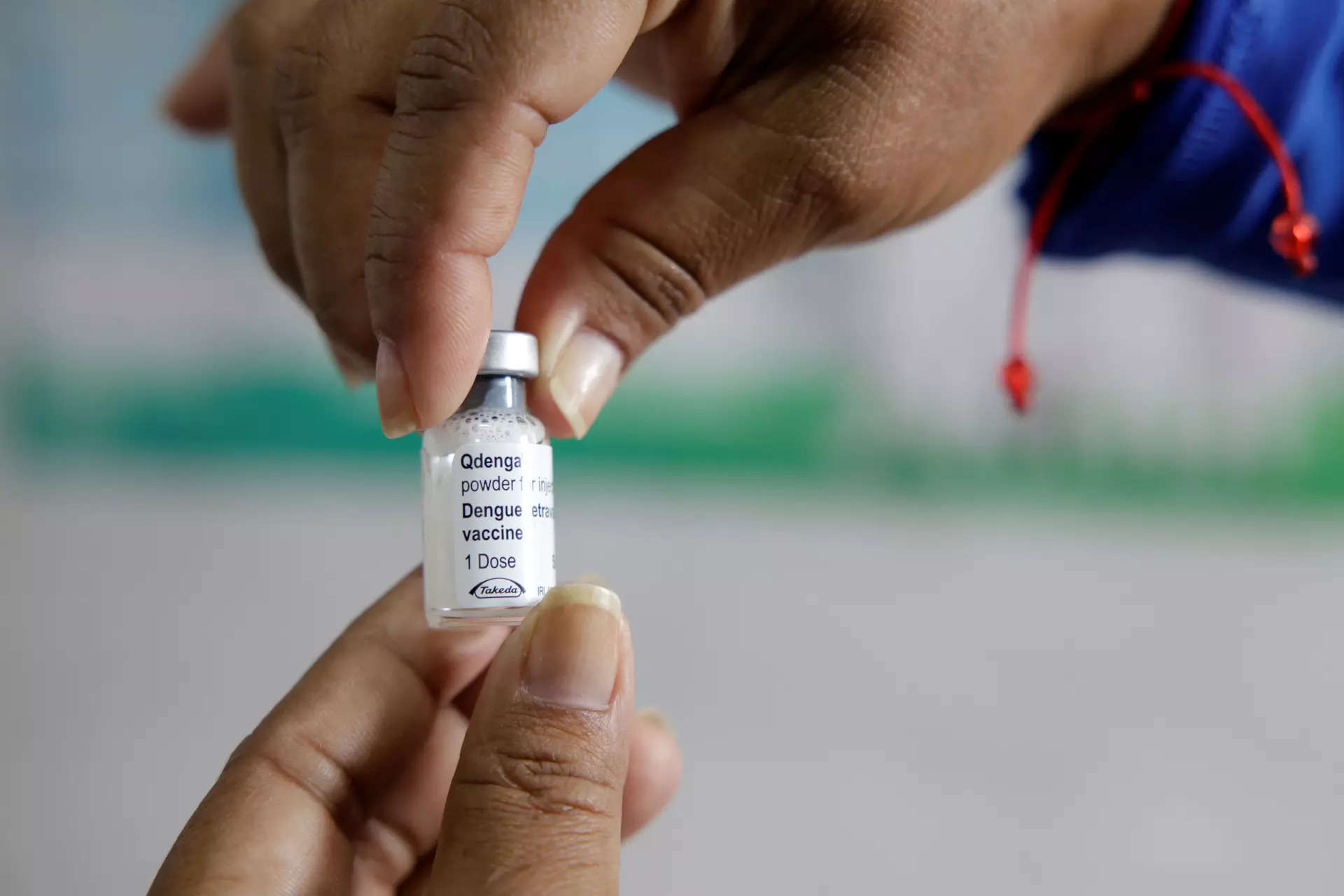
Takeda Pharmaceuticals’ dengue vaccine has been tested globally on about 28,000 participants across phase 1, 2 and 3 trials, including the pivotal phase 3 tetravalent immunisation against dengue efficacy study trial. The vaccine offers protection against all four serotypes of dengue virus, regardless of prior dengue serotype status. It has prevented hospitalised dengue with 84% efficacy.
The vaccine has been approved in more than 30 countries, including the UK, Brazil, Argentina, Indonesia, Thailand, Malaysia and in Europe.Gary Dubin, Hyderabad-based president of the Global Vaccine Business Unit at Takeda Pharmaceuticals, said the company is gearing up to manufacture at least 100 million doses per annum of the dengue vaccine by the end of 2030.As part of this plan, the company early this week announced a manufacturing partnership with Indian vaccine maker Biological E (BE), which will produce up to 50 million doses of the vaccine per annum.
“We’re currently working with a contract manufacturing organisation in Germany, and we have built our own manufacturing facility, also in Germany. But together those two sources will still not approach the 100 million doses (per annum) that we are targeting by the end of the decade. That’s because the vaccine is needed in many countries and a large supply is essential,” Dubin said. “Biological E is a critical part of that strategy and will ultimately supply about half of our total capacity.”
Dubin said Takeda Pharmaceuticals is expanding its vaccine pipeline through partnerships, with a focus on norovirus and ika vaccines.