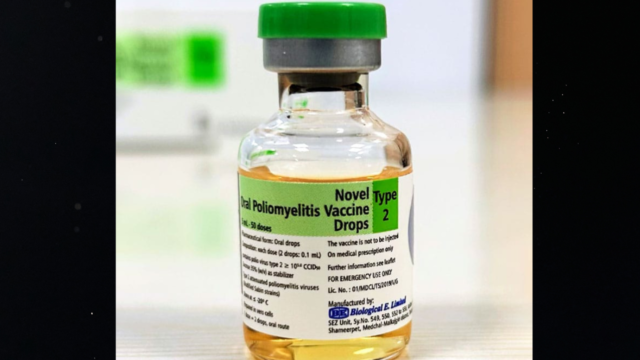
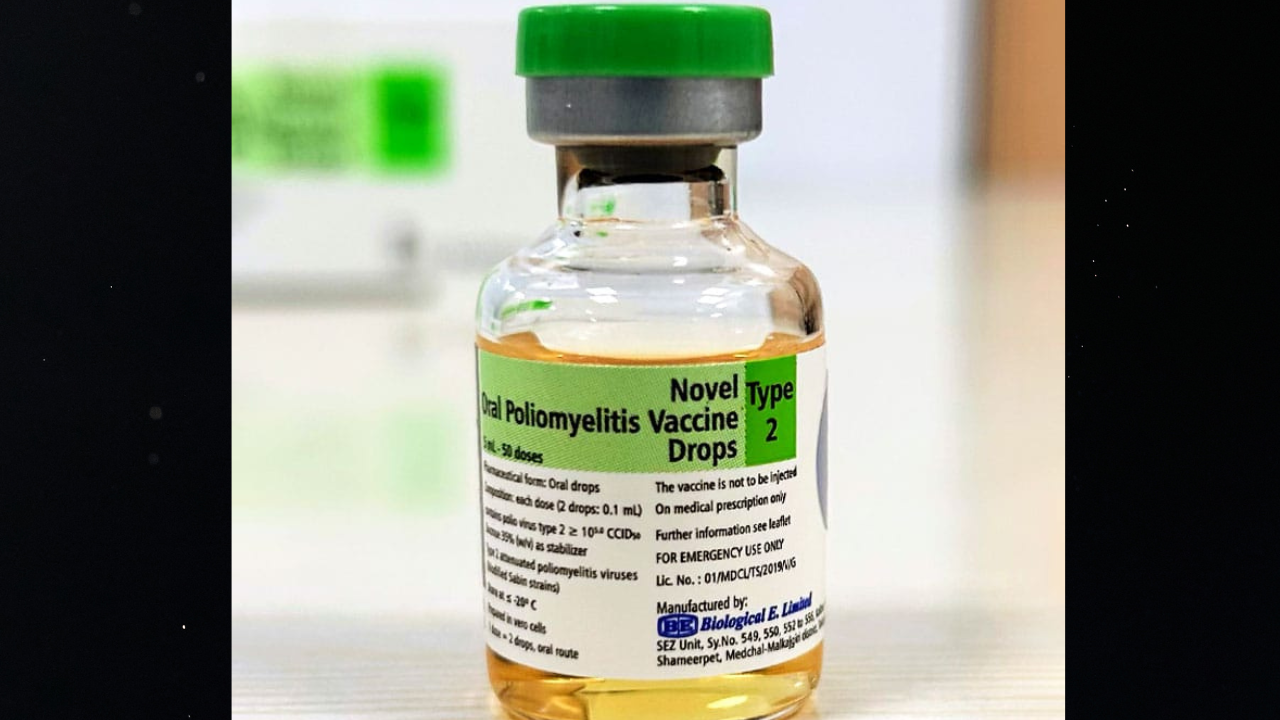
HYDERABAD: Biopharma player Biological E Limited (BE) has received World Health Organisation (WHO) Pre-qualification (PQ) status for its Novel Oral Polio Vaccine type 2 (nOPV2), a next-generation live, attenuated oral vaccine.
The WHO prequalification for nOPV2 makes it the 10th vaccine from Hyderabad-based BioE to get pre-qualified.
BE said extensive clinical trials rigorously evaluated the safety and immunogenicity of nOPV2 with promising results that have been published in reputed medical journal The Lancet (2019-2024).
The oral polio vaccine significantly reduces the risk of circulating vaccine-derived Poliovirus type 2 (cVDPV2) outbreaks and is aimed at immunisation in countries that are affected by cVDPV2 outbreaks, BE said on Tuesday.
“With its improved genetic stability, nOPV2 has a significantly decreased chance of seeding new outbreaks in low-immunity environments as against its predecessor, the Sabin poliovirus type 2 (mOPV2) vaccine,” BE said.
According to the company, the vaccine’s real-world deployment in outbreak regions has shown that it can significantly decrease the incidence of cVDPV2 outbreaks.
Terming the WHO PQ for the vaccine as a significant milestone, BE managing director Mahima Datla said the vaccine has been specifically designed to address concerns about Vaccine-Associated Paralytic Polio (VAPP), which has been found to occur in around two to four cases per million births with the traditional OPV due to the vaccine virus reverting to a virulent form.
BE said it has become an important player in the production of the nOPV2 vaccine and has been selected for a grant from the Bill and Melinda Gates Foundation (BMGF) to assist in meeting the growing global demand for the vaccine for polio eradication.
The company has received technology for the vaccine from PT BioFarma of Indonesia, which is the first manufacturer of the nOPV2 vaccine to receive WHO prequalification in January 2024, and has the capacity to produce over 500 million doses of the vaccine annually.
The company said it has also received approval from the Indian regulatory authorities to manufacture the vaccine for exports.
The WHO prequalification for nOPV2 makes it the 10th vaccine from Hyderabad-based BioE to get pre-qualified.
BE said extensive clinical trials rigorously evaluated the safety and immunogenicity of nOPV2 with promising results that have been published in reputed medical journal The Lancet (2019-2024).
The oral polio vaccine significantly reduces the risk of circulating vaccine-derived Poliovirus type 2 (cVDPV2) outbreaks and is aimed at immunisation in countries that are affected by cVDPV2 outbreaks, BE said on Tuesday.
“With its improved genetic stability, nOPV2 has a significantly decreased chance of seeding new outbreaks in low-immunity environments as against its predecessor, the Sabin poliovirus type 2 (mOPV2) vaccine,” BE said.
According to the company, the vaccine’s real-world deployment in outbreak regions has shown that it can significantly decrease the incidence of cVDPV2 outbreaks.
Terming the WHO PQ for the vaccine as a significant milestone, BE managing director Mahima Datla said the vaccine has been specifically designed to address concerns about Vaccine-Associated Paralytic Polio (VAPP), which has been found to occur in around two to four cases per million births with the traditional OPV due to the vaccine virus reverting to a virulent form.
BE said it has become an important player in the production of the nOPV2 vaccine and has been selected for a grant from the Bill and Melinda Gates Foundation (BMGF) to assist in meeting the growing global demand for the vaccine for polio eradication.
The company has received technology for the vaccine from PT BioFarma of Indonesia, which is the first manufacturer of the nOPV2 vaccine to receive WHO prequalification in January 2024, and has the capacity to produce over 500 million doses of the vaccine annually.
The company said it has also received approval from the Indian regulatory authorities to manufacture the vaccine for exports.