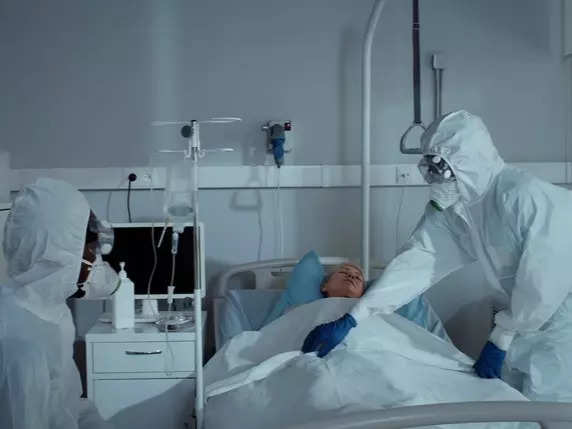
Amid the recent concerns regarding potential rare side effects of the AstraZeneca-Oxford COVID-19 vaccine, the pharmaceutical giant has reaffirmed its dedication to patient safety. It underscored the vaccine’s overall safety profile while prioritising the well-being of recipients.
An AstraZeneca spokesperson stated, “Our sympathy goes out to anyone who has lost loved ones or reported health problems. Patient safety is our highest priority, and regulatory authorities have clear and stringent standards to ensure the safe use of all medicines, including vaccines.”
The pharmaceutical company maintained that the vaccine’s safety and efficacy are consistently supported by extensive clinical trial data and real-world evidence. Regulatory agencies worldwide continue to state that the benefits of vaccination are greater than the risks of these extremely rare side effects.
Earlier this week, AstraZeneca admitted that its Covid-19 vaccine can cause a rare side effect called Thrombosis with Thrombocytopenia Syndrome (TTS). This acknowledgment coincides with the company being sued for claims of serious harm and fatalities linked to the vaccine.
AstraZeneca collaborated with the University of Oxford to develop the vaccine and is currently involved in legal proceedings initiated by victims and their families. One particular case includes Jamie Scott, a father of two, who took legal action after experiencing a blood clot that rendered him unable to work. Scott claims that following his vaccination in April 2021, he developed a “blood clot and a bleed on his brain,” resulting in a lasting brain injury.According to multiple reports from UK media, AstraZeneca has acknowledged in court papers related to a lawsuit claiming that the vaccine, created in partnership with the University of Oxford, resulted in fatalities and severe harm in numerous instances.AstraZeneca had also partnered with the Serum Institute of India (SII), the world’s largest vaccine producer, to supply the vaccine to the Indian Government.
The Serum Institute of India has developed the Covishield without utilizing the mRNA platform. The vaccine was created using the viral vector platform. The vaccine involved modifying a chimpanzee adenovirus – ChAdOx1 – to transport the COVID-19 spike protein into human cells. Although this cold virus cannot infect the recipient, it effectively instructs the immune system to develop a defense mechanism against similar viruses.
The same technology was utilised for creating vaccines for viruses such as Ebola.
Meanwhile, ET reported on May 1 that the parents of Karunya, a data scientist student who allegedly died after taking Covishield in 2021, have decided to file a case against Serum Institute of India (SII). This decision came a day after AstraZeneca, which sold the vaccine in India, admitted in court that their Covid shot can cause a rare side effect.
Venugopalan Govindan, father of Karunya, said the admission by AstraZeneca is “too late’ and has come after so many lives have been lost. “AstraZeneca and SII should have stopped the manufacture and supply of these vaccines when 15 European countries either suspended or age-limited these due to deaths from blood clots that happened in March 2021, within a couple of months of the rollout of the vaccine itself.”
Notably in 2023, the World Health Organisation (WHO) said in its report that TTS emerged as a new adverse event following immunisation in individuals vaccinated with COVID-19 non-replicant adenovirus vector-based vaccines.
This sentence refers to the AstraZeneca COVID-19 ChAdOx-1 vaccine and the Johnson & Johnson Janssen COVID-19 Ad26.COV2-S vaccines.
“TTS is a serious and life-threatening adverse event. WHO has issued this interim emergency guidance to increase awareness about TTS in the context of COVID-19 vaccination and help healthcare providers in the assessment and management of potential TTS cases,” the 2023 statement by WHO read.
Union Health Minister Mansukh Mandaviya in March 2024 at “Dialogues – Navigating India’s health sector’ said that ICMR has done a detailed study which shows that COVID-19 vaccine is not responsible for heart attacks, and an individual’s lifestyle and factors such as binge drinking could be among underlying causes.
Mandaviya said, “If someone has a stroke today, they think it is because of the Covid vaccine. ICMR has done a detailed study that the (Covid) vaccine is not responsible for heart attacks.”
(with ANI inputs)